tags:
- chem
topic: Redox
date: 2023-10-31Introduction
key concepts
- In galvanic cells, electrons flow through the external circuit from the anode to the cathode. (electrons, external)
- Ions carry electrical charge through the internal circuit (cations to cathode and anions to anode) (ions, internal)
- The more reactive metal is located at the anode, as this is where electrons are lost.
- Electrochemical cells facilitate electron transfer in spontaneous redox reactions between oxidisers and reducers in order to convert energy into a usable form.
- Two kinds of electrochemical cells: galvanic and electrolytic.
- Galvanic cells:: Electrochemical cells which convert chemical energy into electrical energy
- Electrolytic cells:: Electrochemical cells which convert electrical energy into chemical energy.
- This requires an external energy supply (electricity) to 'force' chemical reactions to occur.
- Galvanic cells produce electrical energy from redox reactions, and thus, are commonly used in batteries.
Galvanic Cells
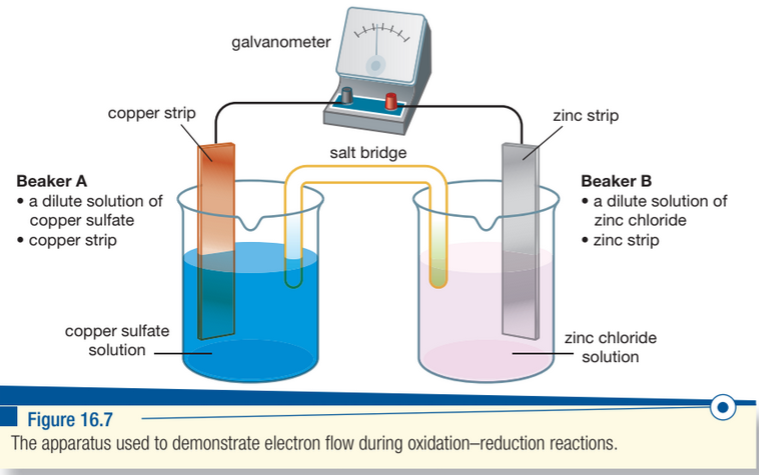
- Three types of galvanic cells:
- Metal half cells [voltaic cells with metal (aka 'active') electrodes]
- Solution half cells [cells with inert (aka 'inactive') electrodes]
- Fuel cells
- Consist of two 'half' cells, with each containing an electrode and electrolyte.
- Electrode:: 'Conductor' with delocalised electrons
- Electrolyte:: The solution that the electrode is 'dipped' within (the metal ions of the electrode as a soluble salt). This solution conducts electricity.
- The electrodes are separate, but are connected by a 'salt bridge' (a piece of filter paper dipped in a solution of a salt) - this salt bridge is usually KCl or NaCl.
- The ions travel through this salt bridge (ions, not electrons)
- As ions carry charge, having the salt bridge with ions increases the charge carried through.
- ANOILRIGCAT: Oxidation happens at the anode, reduction happens as the cathode.
- The ions travel through this salt bridge (ions, not electrons)
- The galvanometer can also be an ammeter or voltmeter.
- Electron flow is determined by referring to the metal reactivity series - the more reactive metal in this series.
- Oxidation only occurs at the anode (-), and the electrons which are lost at this site transfer to the electrode at the cathode across the external (upper) circuit.
- Reduction only occurs at the cathode (+), and the electrode accepts the transferred electrons (therefore being reduced).
- Electrons always move from the anode to the cathode.
- The ions from the two solutions shift to their respective sides; cations to the cathode, and anions to the anode in the internal circuit/salt bridge.
Galvanic Cell Reactions
At the anode in the above image, the following oxidation reaction is occurring:
At the cathode in the above image, the following reduction reaction is occurring:
Over time, there is an eventual formation of pure copper substance at the cathode, and the mass of the zinc at the anode will decrease.
Galvanic Cell Summary Table
| Property | Anode | Cathode |
|---|---|---|
| Electron flow | Out | In |
| Ion flow | Negative (anion) | Positive (cation) |
| Agent | Reducing agent | Oxidising agent |
| Charge | Negative | Positive |
| Redox | Oxidation | Reduction |
Electrolytic Cells
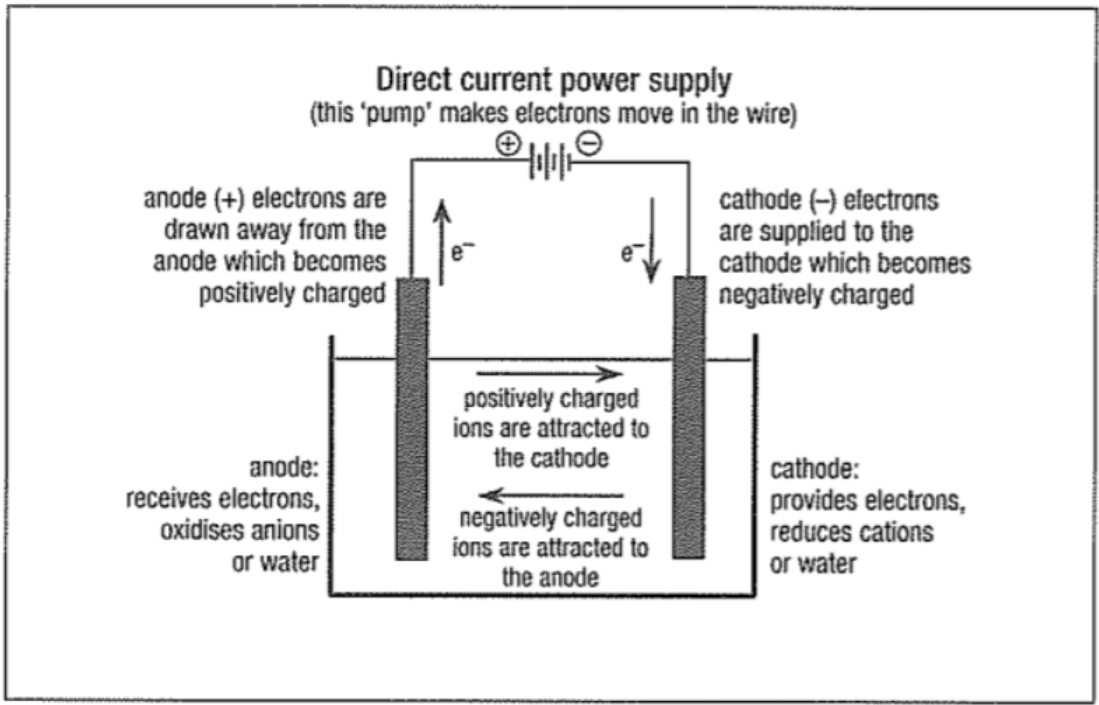
- Electrolysis is the inverse of the redox process, in which electrical energy is converted to chemical energy to power chemical reactions that may not occur otherwise.
- This process obviously requires electricity to occur (hence why electrolytic cells are so expensive)
- The anode and cathode are connected via a power supply, with the positive end of the power supply (the lead) connected to the anode.
- Both the anode and cathode are located in the same container - they share an electrolyte.
- There is another primary difference between electrolytic cells - the anode is positively charged, and the cathode is negatively charged.
- Ion flow (as in the electrolyte) does not change.
- Electrolytic cells are often used to decoratively electroplate cutlery, such as spoons, with a metal.
Electrolytic Cell Reactions
Water electrolysis
- Electrolysis of water will break down water into its corresponding parts - oxygen gas at the anode, and hydrogen gas at the cathode.
- Anode:
- Cathode:
Molten electrolysis
- Electrolysis of molten
- This is in the presence of zero water, otherwise the reaction may be impacted.
- Anode:
- Cathode:
- This is a special case in which hydroxide ions are used to balance the equation, not hydrogen. ↩︎
Interactive Graph